Past Issues
Cerebral Abscess: A Complex Complication of Odontogenic Infection: A Case Report and Review of the Literature
Daniels JS1, AlBakry I1, Braimah RO2*, Samara MI3
¹Consultant, Department of Maxillofacial Surgery, King Khalid Hospital, Najran Kingdom of Saudi Arabia 2Specialist, Department of Maxillofacial Surgery, Specialty Regional Dental Center, Najran, Kingdom of Saudi Arabia 3Specialist, Department of Maxillofacial Surgery, King Khalid Hospital, Najran, Kingdom of Saudi Arabia
Corresponding author: Braimah Ramat Oyebunmi, Department of Oral and Maxillofacial Surgery, Specialty Regional Dental Center, Najran, Kingdom of Saudi Arabia, Najran Kingdom of Saudi Arabia.
Received: October 03, 2019 Published: October 31, 2019
ABSTRACT
Cerebral abscess is a rare sequela of odontogenic infection. Neglected odontogenic infections can progress to extremely dangerous complications including; cellulitis, myofascial space abscess, osteomyelitis, lymphadenitis, bacteremia or sepsis and intracranial abscess. It is usually seen in immunocompromised patients however cases have been reported in immunocompetent patients. Intracranial spread of infection involves complex mechanisms and the following routes of spread have been well-documented; contiguous spread (otitis media, dental infection, mastoiditis, sinusitis). It can result from direct contiguous spread, hematological, lymphatics and direct inoculation from trauma or neurosurgery. Idiopathic or cryptogenic cause has also been reported in 15% of cases. Computed Tomographic (CT) scan and Magnetic Resonance Imaging (MRI) are standard modalities of investigating these lesions. However, MRI is more sensitive than CT scan especially in the early cerebritis stage where CT scan with contrast may be normal. We report a case of cerebral abscess of odontogenic origin in a previously undiagnosed diabetic patient that was seen and managed in our health facility.
Keywords: Abscess; Immunocompromised; Cerebral; Odontogenic.
INTRODUCTION
Cerebral abscess of odontogenic origin is uncommon and only accounts for a few of reported cases of cerebral abscesses [1]. The reported incidence cerebral abscess ranges from 0.4 to 0.9 cases per 100,000 people [1]. Brain abscesses represent about 1% to 2% of all intracranial masses and odontogenic cerebral abscess range between 3% to 10% of all cases of brain abscess [2].
Immunocompromised patients and those with other medical predisposition are seen more frequently with this condition. It has been reported that oral flora are found in about 3% to 10% of all cases of intracranial abscess, while the other causes include non-oral bacteria, Mycobacterium tuberculosis, fungi and parasites [2].
Intracranial spread of infection involves complex mechanisms and the following routes of spread have been welldocumented; contiguous spread (otitis media, dental infection, mastoiditis, sinusitis), hematological [3], lymphatics [4] and direct inoculation during a trauma or neurosurgery [5,6]. Direct contiguous spread have been reported in about 40% to 50% of cases [7], while hematological spread is seen in 25% of cases [8,9] and 10% seen in trauma [10]. Idiopathic or cryptogenic cause has also been reported in 15% of cases[11]. Direct spread tends to cause solitary brain abscess whereas haematogenous spread usually results in multiple brain abscesses [12]. Computed Tomographic (CT) scan and Magnetic Resonance Imaging (MRI) are standard modalities of investigations [13].
Many reported cases of odontogenic brain abscesses did not have active dental focus of infection and have only been arrived at after a method of elimination or exclusion of other common causes [14]. We report a case of cerebral abscess secondary to an active odontogenic infection. To the best of our knowledge this is one of the few reported cases of brain abscess resulting from an active odontogenic infection and the first report of such rare clinical entity from our region.
CASE REPORT
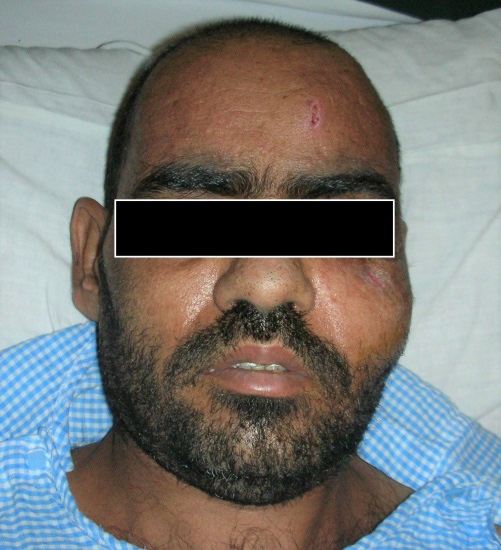
A 30-year-old male Yemeni patient presented to the emergency unit with painful left facial swelling involving the cheek, periorbital area, trismus, fever, reduced hearing in the left ear and inability to see with the left eye due to the facial swelling. The swelling was first notice a week before he reported to the hospital. He gave previous history of dental pain preceding the swelling. His medical history was unremarkable with no known allergy. He was not a known diabetic patient.
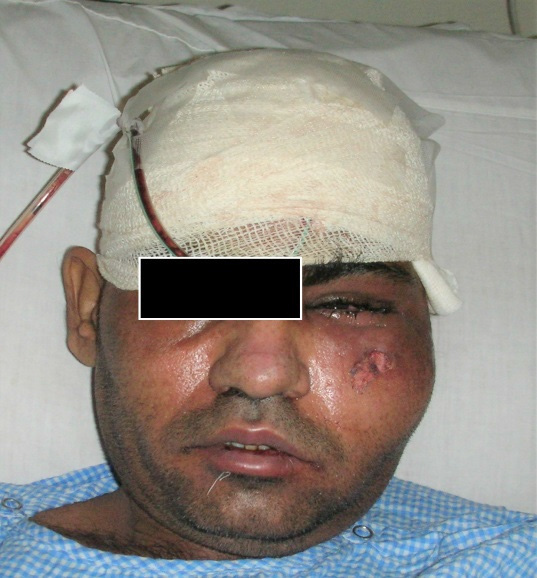
On examination, the patient was alert and fully conscious and exhibited no weakness. There was a huge, diffuse and tender swelling on the left side of the face extending from the lateral aspect of the mandible to the left temporal region as well as a tense left circumorbital swelling preventing the eye from opening (Figure 1). Intraorally, he had severe trismus, very poor oral hygiene with grossly carious upper left second premolar and first molar teeth.
Figure 1: Clinical photograph of the patient at presentation showing swelling of the left side of face with circumorbital edema.
His vital signs on arrival were: Temperature 38.5 ºC, pulse rate 108/min, blood pressure 137/83, respiratory rate 18/min. Full blood count showed leukocytosis 20 × 103 /uL and absolute neutropenia with left shift, hemoglobin was 12.1 gm/dL and blood group was A positive. Blood chemistry showed normal values for electrolytes, urea and creatinine, fasting blood sugar was 9.34 mmol/L and a high glycated hemoglobin level of 6.4% (suggesting diabetes), low total protein of 64 g/l (64-82) and serum albumin of 30 g/l (34-50). Erythrocyte sedimentation rate was abnormally high (88 mm/hr). Serology, including HIV test, was negative. A provisional diagnosis of left facial cellulitis in a previously unknown diabetic patient was made. Empirical intravenous augmentin 1.2 g TID, Metronidazole 500 mg TID and dexamethasone 8mg stat followed by 4 mg QID was commenced. He was admitted under the care of the maxillofacial surgeons and reviewed by ophthalmologists, and endocrinologists. The endocrinologist put the patient on diabetic diet and an insulin sliding scale.
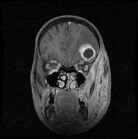
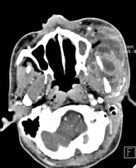
CT scan done with intravenous contrast at the time of admission showed a large left-sided multiloculated facial collection containing gas locules extending from the mandible to the pterygoid fossa, the infratemporal fossa with periorbital preseptal extension as well as mucosal thickening of the left maxillary sinus (Figure 2a and 2b).
Figure 2A: CT Scan showing pus collection in left maxillary sinus, buccal and infratemporal regions in axial view.
Figure 2B: CT Scan showing pus collection the left buccal, temporal and infratemporal regions in coronal view.
The carious upper left second premolar and first molar teeth were extracted and incision and drainage of the left buccal abscess with insertion of corrugated drain under local anaesthesia. The culture and sensitivity report showed growth of Streptococcus spp. sensitive to Penicillins, Klebseilla pneumoniae sensitive to Cephalosporins. Ceftriaxone 1.0 g i.v. TID was thereafter added to his medication.
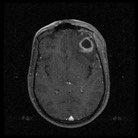
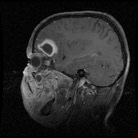
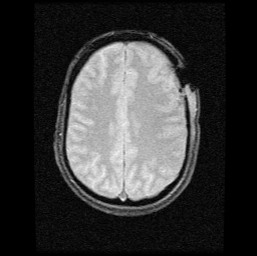
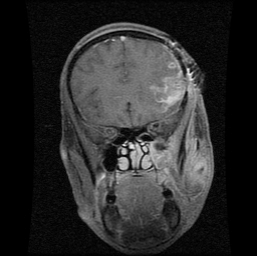
The facial swelling was improving albeit slowly and his mouth opening was satisfactory. Repeat CT scan showed improvement in the facial swelling and no lesion in the brain. About three days later the patient started complaining of headache and showing signs of apathy and malaise but no weakness. Subsequent full blood count done a week later revealed a marked leukocytosis with increased white cell count to 23.47 × 10 3 /uL even though the facial swelling was improving. MRI was done and it showed pus collection in the left maxillary sinus, buccal, temporal and infratemporal, orbital regions with intracranial extension into frontal lobe and mild right shift of midline structures (Figure 3a-3c). However, he had no neurological deficit but showed some level of altered sensorium.
Figure 3a: MRI showing pus collection in frontal lobe in axial view.
Figure 3b: MRI showing pus collection in frontal lobe in sagittal view.
Figure 3C: MRI showing pus collection in frontal lobe in coronal view with buccal, infratemporal and temporal regions.
He was taken back to the operating theatre by a combined team of maxillofacial surgeons and neurosurgeons for drainage of the residual buccal abscess and left maxillary empyema via Caldwell-Luc approach by maxillofacial surgeons and drainage of the frontal lobe abscess by craniotomy through temporofrontal approach by the neurosurgeons respectively, under general anesthesia (Figure 4). Culture and sensitivity of the intracranial abscess was negative. Post craniotomy MRI showed relieved facial and frontal lobe abscess (Figure 5a and 5b). Our patient recovered fully with no neurological deficit after 53 days of hospital admission (Figure 6).
Figure 4: Clinical photograph showing patient at immediate postoperative incision and drainage via craniotomy with vacuum drain.
Figure 5a: Post craniotomy MRI showing resolution of frontal lobe abscess in axial view.
Figure 5b: Post craniotomy MRI showing resolution of frontal lobe abscess in coronal view.
Figure 6: Clinical photograph showing patient on discharge from hospital.
DISCUSSION
Neglected odontogenic infections can progress to extremely dangerous complications including; cellulitis, myofascial space abscess, osteomyelitis, lymphadenitis, bacteremia or sepsis and intracranial abscess [15]. Intracranial abscess is a universal health problem and had a high morbidity and mortality rate (up to 80%) especially in pre-antibiotic and inadequate radiodiagnostic era [13,16]. Lately, the advances in neuro-scanning techniques such as CT and MRI, as well as the availability of more effective antibiotics, have reduced the mortality rate to 0% to 24% [17]. Odontogenic cerebral abscess affects more males than females by a margin of approximately 82.1% and usually occurs in the 5th and 6th decades of life [11,18] . The case presented here is unusual because, at the age of 30 years, our patient was younger than the usual age of presentation. It has been reported that age and underlying immune status are risk factors for the development of intracranial abscess [11].
The most common aetiological organisms of odontogenic brain abscess reported in the literature include microaerophilic streptococci (Streptococci viridans), anaerobic bacteria (Bacteroides spp., Actinobacillus actinomycetemcomitans), Staphylococcus aureus, and facultative anaerobic Gram negative bacteria (Enterobacteriaceae) [3]. Haggerty & Tender [19] reported a case of actinomycotic brain abscess following endodontic treatment of anterior maxillary teeth. In our patient, both Streptococci spp. and Klebsiela pneumoniae were isolated.
Klebseilla pneumoniae infection is seen in patients with compromised immune system especially diabetes [3]. Although our patient was not a previously known diabetic, investigations revealed otherwise as patient had poor glycemic control. High index of suspicion of an underlined medical condition is important in patients with spreading craniofacial infection.
Immunodeficiency secondary to HIV infections, alcohol abuse, diabetes, chemotherapy or malignancy can promote the development of brain abscess [20]. However, several cases of brain abscess have been described in otherwise healthy patients with poor oral hygiene or after dental treatment [20,21].
Another poorly reported etiology of compromised immune status is the issue of chronic malnutrition [22]. Our patient suffered from hypoproteinemia with total protein and albumin below normal values. Glutamine and arginine are important amino acid that is necessary for optimal immunologic cell function and immune modulation [23]. Reduction in total body protein and albumin can affect this important immune function [14]. The concept of chronic malnutrition in the spread of odontogenic infections have been reported by Braimah et al. [22,24] in Sokoto, northwestern Nigeria where many people live below poverty line.
It has been reported that odontogenic infections, ethmoidal or frontal sinusitis usually spread to the frontal lobe while sub-acute or chronic otitis media or mastoiditis spread to the inferior temporal lobe and cerebellum [13]. Our case further validates the literature finding as the spread was into the frontal lobe, however temporo-parietal intracranial spread from an odontogenic infection has been reported by Hibberd et al. [25] in a pediatric patient. Brain abscesses are rare in children and are most likely to occur within the first decade of life because of the higher prevalence of sinus and middle ear infections [26]. We opined that perhaps middle ear infection which is more common in this age group might have coincided with the dental infection.
Computed Tomographic (CT) scan and Magnetic Resonance Imaging (MRI) are standard modalities of investigations [13]. However, MRI is more sensitive than CT scan especially in the early cerebritis stage where CT scan with contrast may be normal [14]. If untreated, cerebritis will proceed to brain abscess in one to two weeks [14] and will show as a spaceoccupying lesion with enhanced rim often surrounded by an area of oedema [12]. The first radiological sign of brain abscess can be seen on CT scan 2-3 weeks after the infection, which is seen on contrast enhanced CT scan as a radiopaque ring surrounding a central necrotic region[12].
Odontogenic infections rarely result in intracranial infection. When they do, haematological spread is considered the most important mechanism [27]. Cerebral abscess caused by odontogenic infection has neither a predilection for laterality and can occur on contralateral side nor predilection for location such as mandible or maxilla since it is mainly transmitted by haematological spread, more by bacteremia than venous spread [27,28] and can develop multiple brain abscesses [20].
Clinically, there is usually a latent period of several days to several weeks after the dental episode before the appearance of intracranial involvement and the initial complaints may be mild consisting of headaches, malaise and apathy [12]. Most patients with brain abscess of odontogenic origin present with neurological symptoms such as epileptic fit [3], hemiparesis and fit [17], progressive weakness in leg and speech difficulties (due to actinomycosis from endodontic treatment) [19], acute onset of leg weakness after endodontic treatment [20], right leg weakness [28]. Although, our patient did not present with neurological deficit, he had some level of altered sensorium which improved following craniotomy. Greenstein et al. [21], reported a case of abscess of the temporal lobe in continuity with a temporal abscess in a patient who had left facial swelling of odontogenic origin, the isolated organism was Staphylococcus epidermidis which was sensitive to vancomycin and ceftriaxone. Bonardi et al. [18], reported a case of brain abscess potentially secondary to periodontal infection resulting in death of a 17-year-old female patient.
Very often, there is no direct evidence that brain abscess arose from odontogenic focus (because many reported cases did not have active dental focus of infection [14,27,28]. Most of the reported cases of odontogenic cerebral abscesses usually present initially to hospital with neurological symptoms long after the suspected odontogenic causes have been treated. Therefore the diagnosis of cerebral abscess as a direct result of dental infection has historically been one of exclusion or elimination of other sources such as sinusitis and otitis media or other common causes with some cases being attributed to dental infection simply because there was dental focus or treatment preceding the development of the cerebral abscess [14,18,27,28]. This has often raised the question if orofacial infections really have the capacity of spreading intracranially, ultimately causing brain abscess [14]. So the most reliable way to confirm the dental aetiology of brain abscess is by matching the culture results of the bacterial samples from both the intracranial and extracranial sites of infection with DNA analysis [14,27,29].
Brain abscess of odontogenic origin may not show any bacteria. One of the reasons may be that many oral bacteria are fastidious and therefore are difficult to culture and any specimen has to be taken to the laboratory under strict anaerobic condition so standard bacterial culture may not serve as pathogen identification [27,30].
Once the diagnosis of brain abscess has been made, treatment consists of three steps namely: administration of antibiotics, drainage of abscess and treatment of the primary focus [28]. The antibiotic treatment is by rapid initiation of intravenous antibiotics. Since the infection may be polymicrobial, an initial combination of antibiotics with extended spectrum of activity should be given as the responsible organisms are not known at the beginning of treatment. This can be modified after the causative organism has been identified. The usual empirical regime consists of a third generation Cephalosporin and Metronidazole. Vancomycin may be added in postsurgical or posttraumatic abscess which are infected with multidrug resistant organism [14,20].
Our management protocol has followed the sequence of resuscitation, identifying underlying the medical condition(s), removal of source of infection, empirical antibiotics and surgery (incision and drainage and craniotomy). The choice of Augmentin, a Penicillin-based antibiotic, was based on the sensitivity of streptococcus to this medication. Common anaerobes are also sensitive to Metronidazole [15] which was also a medication of choice in the present case. Ceftriaxone, a beta-lactam of the third generation Cephalosporins, was added to the medication regimen when microbiology results showed its sensitivity to Klebseilla.
The use of steroids has been controversial as Saldago et al. [15] reported that its administration should be generally avoided unless the patient demonstrates signs of meningitis or disproportionate cytotoxic edema posing a life threatening clinical condition. Others have recommended that the management includes intravenous glucocorticoids together with intravenous 3rd generation Cephalosporin such as Ceftriaxone in combination with Metronidazole.in patients with evidence of mass effect from brain abscess [12]. This patient was given steroids as the cranial spread was deemed dangerous to his health. The authors were not surprised that intracranial abscess microbiology was negative, as patient had been on antibiotics for a while. However, positive brain cultures have been reported in a case of recurrent brain abscess secondary to dental infection [25].
CONCLUSION
Intracranial abscess formation associated with an odontogenic source is a rare occurrence and its clinical features are often nonspecific. Most of the reported cases of odontogenic cerebral abscesses usually report initially to hospital with neurological symptoms long after the suspected odontogenic causes have been treated and their association have only been established after elimination or exclusion of other sources such as sinusitis, otitis media or exclusion and not evidence-based. Our patient is one of the few reported cases which presented with active odontogenic abscess and progressed to cause brain abscess. Oral health care providers should therefore be more vigilant in identifying and prompt treatment of dental conditions so as to prevent intracranial complications.
Conflicts of Interest
Author JSD, Author IA, Author MIS, Author ROB declare that they have no conflict of interest.
REFERENCES
- Van der Cruyssen F, Grisar K, Maes H, Politis C (2017) Case of cerebral abscess caused by Polyphromonas gingivalis in a subject with periodontitis. BMJ Case Rep pii: bcr2016218845.
- Clifton TC, Kalamchi SA (2012) A case of odontogenic brain abscess arising from covert dental sepsis. Ann R Coll Surg Engl 24(1): 41-43.
- Corson M, Posthlewaite KP, Seymour RA (2001) Are dental infections a cause of brain abscess? Case report and review of the literature. Oral Dis 7(1): 61-65.
- Li X, Tronstad L, Olsen I (1999) Brain abscess caused oral infection. Endod Dent Traumatol 15(3): 95-101.
- Azenha MR, Homsi G, Garcia IR (2011) Multiple brain abscess from dental origin. Case report and literature review. Oral Maxillofac Surg 16(4): 393-397.
- Sheehan JP, Jane JA, Ray DK, Goodkin HP (2008) Brain abscess in children. Neurosurg Focus. 24(6): E6.
- Glickstein JS, Chandra RK, Thompson JW (2006) Intracranial complications of pediatric sinusitis. Otolaryngol Head Neck Surg. 134(5): 733-736.
- Carpenter J, Stapleton S, Holliman R (2007) Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis 26(1): 1-11.
- Singh N, Husain S (2000) Infection of the central nervous system in transplant recipients. Transpl Infect Dis 2(3): 101-111.
- Brouwer MC, Van de Beek D (2017) Epidemiology, diagnosis and treatment of brain abscesses. Curr Opin Infect Dis 30(1): 129-134.
- Helweg-Larsen J, Astradsson A, Richhall H, Erdal J, Laursen A, Brennum J (2012) Pyogenic brain abscess, a 15-year survey. BMC Infect Dis 12: 332.
- Bali RK, Sharma P, Gaba S, Kaur A, Ghanhas PA (2015) Review of complications of odontogenic infections. Natl J Maxillofacial Surg 6(2): 136-143.
- Alvis-Miranda H, Castellar-Leones S, Elzain M, MoscoteSalazar L (2013) Brain abscess Current management. J Neurosci Rural Prac 4(6): 67-81.
- Lazow SK, Izzo SR, Vasquez D (2011) Do dental infection really cause central nervous system infections? Oral Maxillofacial Surg Clin N Am 23(4): 569-578.
- Salgado JM, Hoz SS, Rojas AN, Satyarthee GD, QuintanaPajaro L, Pacheco-Hernandez A (2018) Brain abscess and dental infections. A review. Roman Neurosurg 32(2): 283- 289.
- Siatouni A, Gatzonis S, Sakas D, Mpouras T, Stefanatou M, Boviatsis E (2007) Brain abscess following intracerebral haemorrhage. J Clin Neurosci 14(10): 986-989.
- Mylonas AL, Tzerbos FH, Mihalaki M, Rologis D, Boutsikakis L (2007) Cerebral abscess of odontogenic origin. J CranioMaxillofac Surg 35(1): 63-67.
- Bonardi JP, Silva LF, Momesso GAC, de Lima VN, Pereira RS, Faverani LP (2019) Brain abscess potentially secondary to periodontal infection resulting in death of a young patient. Oral Surgery 12(2): 1-3.
- Haggerty CJ, Tender GC (2012) Actinomycotic brain abscess and subdural empyema of odontogenic origin. Case report and review of the literature. J Oral Maxillofac Surg 70(3): e210-e213.
- Pallesen LP, Schaefer J, Reuner U, Leonhardt H, Engellandt K, Schneider H et al. (2014) Multiple brain abscess in an immunocompetent patient after undergoing professional tooth cleaning. JADA 145(6): 564-568.
- Greenstein A, Witherspoon R, Leinkram D, Malandreni M (2015) An unusual case of a brain abscess arising from an odontogenic infection. Australian Dental J 60(4): 532-535.
- Braimah RO, Taiwo AO, Ibikunle AA (2016) Ludwig’s angina: Analysis of 28 cases seen and managed in Sokoto. Northwest Nigeria. Saudi Surg J 4(2): 77-83.
- Hughes P, Bradrick JP, Yowler CJ (2013) Nutrition for the oral and maxillofacial surgery patient. In Fonseca RJ, Barber HD, Walker RV, Powers MP, Frost DE [Eds], Oral and Maxillofacial Trauma [4th Ed.]. Elsevier Saunders, St Louis, Missouri, USA, 30-47.
- Braimah RO, Ibikunle AA, Taiwo AO, Tunau K (2017) Ludwig’s angina in pregnancy and puerperium. Case series in an academic hospital, Sokoto, Northwest Nigeria. Saudi J health Sci 6(2): 130-133
- Hibberd CE, Nguyen TD (2012) Brain abscess secondary to a Dental Infection in al 11-year-old child. Case report. J Can Dent Assoc 78: 49-55.
- Goodkin HP, harper MB, Pomeroy SL (2004) Intracerebral abscess in children. Historical trends at Children’s Hospital Boston. Pediatrics 113(6): 1765-1770.
- Akashi M, Tanaka K, Kusumoto J, Shungo F, Kohkichi H, Komori T (2017) Brain abscess potentially from odontogenic focus. Report of three cases and a literature Review. J Maxillofac Oral Surg 16(1): 58-64.
- Yang J, Liu SY, Hossaini-Zadeh M, Pogrel MA (2014). Brain abscess potentially secondary to odontogenic infection Case report. Oral Surg Oral Med Oral Pathol Oral Radiol 117(2): 108-111.
- Mueller AA, Saldamli B, Stubinger S, Walter C, Fluckiger U, Zimmerer S (2009) Oral bacterial cultures in non-traumatic brain abscesses. Results of a first-line study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107(4): 469-476.
- Moazzam AA, Rajagopal SM, Sedghizadeh PP, Zada G, Habibian M (2015) Intracranial bacterial infection of oral origin. J Clin Neurosci 22(5): 800-806.
Copyright: Daniels JS, et al. ©2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Daniels JS (2019). Cerebral Abscess: A Complex Complication of Odontogenic Infection: A Case Report and Review of the Literature. Surgeries 1(1): 4.
 Abstract
Abstract  PDF
PDF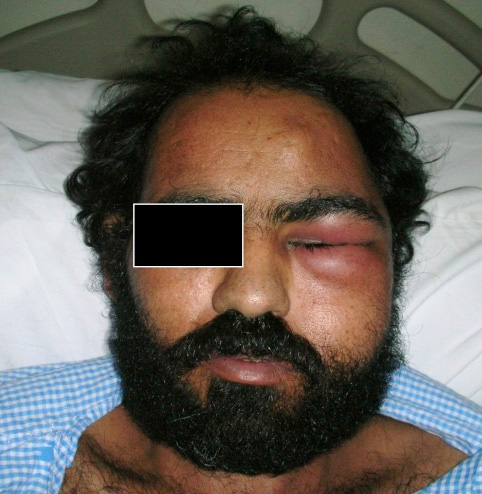
.bmp)